The Science Behind Teeth Whitening
Teeth whitening, a popular cosmetic procedure, hinges on a fascinating interplay of chemical reactions. Understanding the underlying science is key to appreciating how these treatments effectively brighten your smile. The process fundamentally involves the use of specific chemicals designed to break down stain molecules embedded within the enamel and dentin of your teeth. These stains can arise from various sources including coffee, tea, wine, and even certain medications. The efficacy of a whitening treatment depends not only on the chemical composition but also on factors like concentration, contact time, and the overall condition of the teeth.
Hydrogen Peroxide’s Role in Whitening
Hydrogen peroxide is perhaps the most prevalent chemical used in teeth whitening. Its effectiveness stems from its ability to act as an oxidizing agent. When applied to the teeth, hydrogen peroxide penetrates the enamel and dentin, where it encounters the stain molecules. Through a process known as oxidation, the hydrogen peroxide breaks down these larger, complex stain molecules into smaller, less noticeable ones, thereby creating a whitening effect. The concentration of hydrogen peroxide varies depending on the treatment, ranging from low percentages in over-the-counter products to higher concentrations used by dental professionals.
How Hydrogen Peroxide Works

The magic of hydrogen peroxide lies in its instability. When it comes into contact with teeth, it decomposes into water and free radicals. These free radicals are highly reactive and seek out stain molecules, breaking them down through oxidation. The process essentially bleaches the teeth from the inside out. The effectiveness is influenced by the concentration of hydrogen peroxide, the duration of contact, and the application method. This can vary from at-home whitening strips and gels to professional in-office treatments. The speed and degree of whitening depend on the individual’s teeth and the type of stains present.
Concentration and Effectiveness
The concentration of hydrogen peroxide is a critical factor in teeth whitening. Higher concentrations generally lead to quicker results, but they also increase the risk of side effects, such as tooth sensitivity and gum irritation. Over-the-counter products typically contain lower concentrations, ensuring safety for home use, although results may be slower to appear. Professional treatments utilize much higher concentrations, often activated by a special light or laser, to accelerate the whitening process. Dentists carefully monitor the concentration and application to minimize any potential adverse effects, tailoring the treatment to each patient’s needs and sensitivity levels.
Carbamide Peroxide Explained
Carbamide peroxide is another common chemical used in teeth whitening, particularly in at-home treatments. It’s a compound that breaks down into hydrogen peroxide and urea. The urea component helps to buffer the pH, which reduces tooth sensitivity. This makes it a gentler option for some users compared to higher concentrations of hydrogen peroxide alone. Carbamide peroxide is usually found in whitening gels that are applied using custom-fitted trays, allowing for prolonged contact with the teeth. The release of hydrogen peroxide over time provides a sustained whitening effect.
Carbamide Peroxide vs. Hydrogen Peroxide

The key difference lies in their composition and how they release the active whitening agent. Hydrogen peroxide is a more potent oxidizing agent, providing faster results, especially at higher concentrations. Carbamide peroxide, on the other hand, releases hydrogen peroxide more slowly, making it suitable for overnight or extended-use whitening. While hydrogen peroxide might be preferred for quick, in-office treatments, carbamide peroxide is often chosen for at-home convenience. The choice depends on individual preferences, the severity of staining, and the desired whitening speed. The dentist will assess the situation and recommend what suits you best.
Breaking Down the Chemical Reaction
The chemical reaction at the heart of teeth whitening involves oxidation. Stain molecules, which are often complex organic compounds, are broken down by the oxidizing action of hydrogen peroxide. This breakdown transforms the larger, colored stain molecules into smaller, colorless ones. The effectiveness of this process relies on the ability of the chemical to penetrate the tooth’s structure and reach the stain molecules. Factors such as the porosity of the enamel and the presence of other substances within the tooth can influence the efficiency of the reaction. A proper understanding of this process allows for creating more effective and efficient whitening solutions.
Other Whitening Agents to Know
While hydrogen peroxide and carbamide peroxide are the primary players, other chemicals also play a role in teeth whitening. These agents may be used to enhance the whitening process or act as alternatives for those with sensitivities. Understanding these additional components provides a more comprehensive view of the chemistry involved in achieving a brighter smile. They can also influence the overall treatment and its results, contributing to the effectiveness of the whitening procedure.
Sodium Chlorite

Sodium chlorite is sometimes used in whitening products, often as a stabilizer or a contributor to the overall whitening effect. It can release chlorine dioxide, which acts as an oxidizing agent similar to hydrogen peroxide. However, its use is less common than the peroxides, and it may be found in some specialized formulations. Sodium chlorite’s inclusion can affect the overall reaction and the final result, especially when combined with other ingredients, providing a complex chemistry that contributes to a bright, white smile.
The Role of Sodium Chlorite
Sodium chlorite can play multiple roles, depending on the whitening product’s specific formulation. It might assist in the breakdown of stains and also help to stabilize the active whitening agents, extending the product’s shelf life and effectiveness. When combined with other chemicals, sodium chlorite can improve the overall efficacy of the treatment. However, the specific contributions can vary, and it is essential to understand the comprehensive formula of any whitening solution to determine the precise impacts of sodium chlorite.
Sodium Perborate
Sodium perborate is another chemical occasionally used in teeth whitening. It releases hydrogen peroxide when it comes into contact with water, offering a milder alternative to the more concentrated forms. This makes sodium perborate a useful ingredient in certain whitening toothpaste formulations and can contribute to gradual whitening. It often appears in products designed for home use, emphasizing a gentler approach to enhancing the brightness of your teeth, providing a different approach.
The Importance of Sodium Perborate

Sodium perborate provides a milder, more controlled release of hydrogen peroxide, reducing the risk of sensitivity. This is particularly useful for individuals with sensitive teeth or those who prefer a gentler approach to whitening. Its use in specific formulations balances the whitening effect with comfort. Its presence enhances the variety of teeth whitening solutions available and gives individuals with varied dental needs a range of alternatives to choose from. It also provides different chemical reactions.
Understanding Whitening Processes
The mechanics of teeth whitening are complex. They involve chemical reactions and an understanding of how stains form and how the bleaching agents work. Comprehending these underlying processes enables the design of more efficient and safe treatments. Knowledge of the specific chemistry involved in teeth whitening helps to choose the right solutions for particular cases, leading to more effective results. A complete understanding helps us to have a brighter and whiter smile.
Oxidation and Staining
Staining occurs when pigments from food, drinks, and other sources accumulate on and within the enamel. These stains can be extrinsic (on the surface) or intrinsic (within the tooth structure). Whitening treatments utilize oxidation to break down the complex stain molecules. Oxidizing agents like hydrogen peroxide or chlorine dioxide react with the stains, causing them to become colorless or less visible. This process restores the natural brightness of the teeth, creating a desirable cosmetic effect.
The Chemistry of Stain Removal
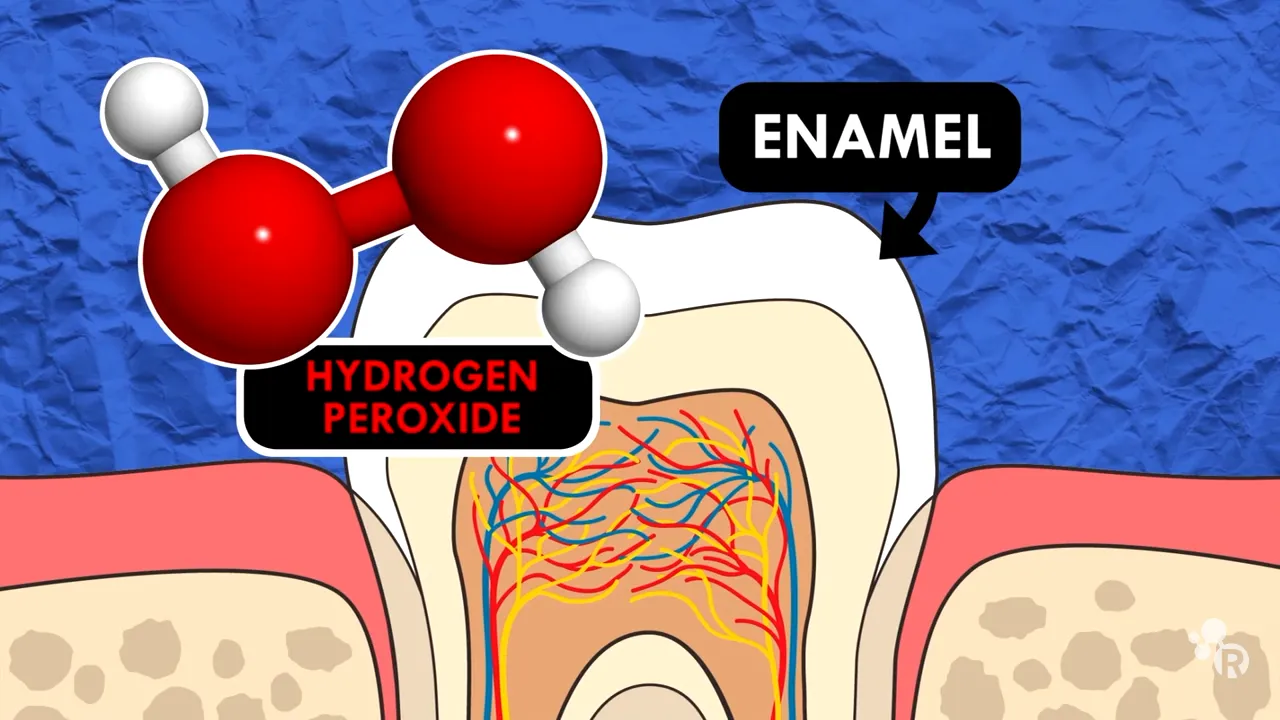
The chemistry of stain removal involves the chemical breakdown of stain molecules. When an oxidizing agent like hydrogen peroxide interacts with stain molecules, it disrupts their structure and converts them into less colored forms. This process essentially ‘bleaches’ the teeth, making them appear whiter. The effectiveness of stain removal depends on factors like the concentration of the oxidizing agent, the contact time, and the type and severity of the stains. Different types of stains might respond differently to the same whitening agent, highlighting the complexity of the process.
Safety and Sensitivity
Tooth sensitivity is a common side effect of teeth whitening. It occurs because the whitening agents can penetrate the enamel and reach the dentin, where they can irritate the nerves. Proper understanding of the chemistry involved allows for managing and reducing the risk of sensitivity. Selecting the right concentration, application method, and duration can help to minimize the chance of adverse reactions. Individuals should also be aware of the possible risks and consult a dentist before undergoing treatment, particularly if they have sensitive teeth or gum issues.
Preventing Tooth Sensitivity
Preventing tooth sensitivity involves several strategies. Using lower concentrations of the whitening agent, applying it for shorter durations, or using products with desensitizing ingredients can help. Products with potassium nitrate can block pain signals to the nerves. It’s also essential to avoid over-whitening and to follow the instructions provided by the dental professional or manufacturer. A thorough consultation with a dentist can provide personalized advice on avoiding or managing tooth sensitivity, ensuring a more comfortable whitening experience.
Conclusion

The chemistry of teeth whitening provides the basis for understanding how we can effectively and safely brighten our smiles. From hydrogen peroxide’s oxidizing properties to the gentler actions of sodium perborate, the science behind teeth whitening is complex and fascinating. Recognizing these chemical interactions is key to making informed decisions about whitening treatments. By understanding these secrets, you can choose the best and safest approach to achieve a radiant, confident smile. Remember to always consult a dental professional for personalized advice.
